Brand History
Dibao was established by company founder, Mr Luo Yongqiang, in 1998, and was officially launched onto the market in the following year. After years of development, Dibao now has a total of 4 categories of IVD reagent products: biochemistry analysis, clinical pathological analysis, dry chemistry analysis, and blood cell analysis. Dibao has its own research team. Through assimilating advanced experience from international companies and undertaking independent research, Dibao is able to expand its product line and improve product quality. Moving forward, Dibao will continue to work with more renowned manufacturers to engage in innovation and provide better products and services to our customers.
Kangdu
Guangzhou KANGDU clinical laboratory was founded in 2003 August, affiliated to HUASIN SCIENCE, obtained the practice license of medical institution of the People's Republic of China. Kangdu was approved by the CHINA NATIONAL ACCREDITATION BOARD FOR LABORATORIES (CNAL) requirements ISO 15189: year 2012
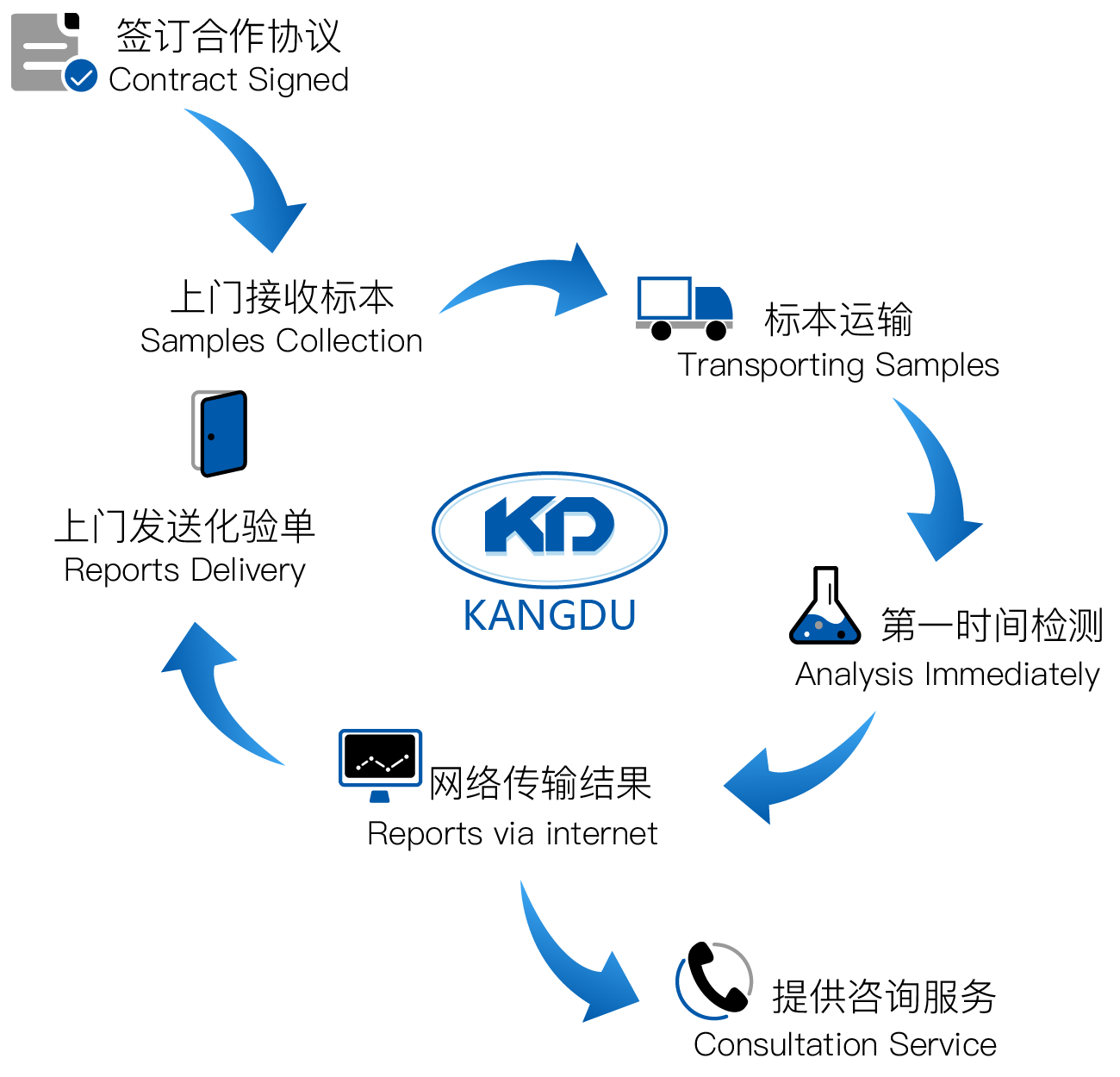
Standardized service process
KangDu Qualification
-

Business license
-

China National Accreditation Service for Conformity Assessment
-

Inter-room quality assessment certificate (clinical microbiology)
-

Inspection and testing institution qualification certificate
-

Laboratory accreditation certificate
-

Inter-room quality assessment certificate (thalassaemia genetic testing)
KangDu honor
-

Inter-room quality assessment certificate (National Infectious Diseases)
-

Inter-room quality assessment certificate (National Whole Blood Five Elements)
-

Inter-room quality assessment certificate (National Nucleic Acid Testing)
-

Inter-room quality assessment certificate (national tumor marker)




